Gas type and pressure
In the case of methane and carbon dioxide,
increasing gas pressure decreases the permeability, but this reduction
is greater for
methane than carbon dioxide. This effect is remarkable at the lower pressures
for both gases, but by increasing the pressure up to 7.5 MPa for carbon
dioxide, the rate of decrease drops further. As for methane, the permeability
decreases to half of its initial amount when the gas pressure increases
from near 0.5 MPa to 2 MPa.
The ratio of CH4/CO2 in Australian coal seams varies; in some deposits
CO2 is the predominant gas, and in other areas methane is predominant.
The permeability of coal is higher for methane than carbon dioxide (Lama,
1995a; Bartosiewicz and Hargraves, 1985). Australian researchers Xue and
Thomas (1995) investigated the permeability of Australian coals to a mixture
of CH4/CO2. They stated that by using the Darcy and Dalton pressure laws,
the permeability of coal to a mixture of two gases can be derived theoretically
from the following Equation:
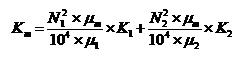
N1
= Volumetric percentage
of gas No 1
N2= Volumetric percentage
of gas No 2
K1= Permeability
to gas No 1, Darcy
K2= Permeability
to gas No 2, Darcy
Km= Permeability
of coal to mixture, Darcy
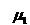 = Viscosity of
gas No. 1, centipoise = Viscosity of
gas No. 1, centipoise
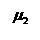 = Viscosity of
gas No. 2, centipoise = Viscosity of
gas No. 2, centipoise
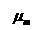 = Viscosity of
mixture gas, centipoise = Viscosity of
mixture gas, centipoise
Xue and Thomas (1995) found the difference between the theoretical calculated
and the experimentally measured permeability was about +/-15%. They also
reported that by increasing the proportion of carbon dioxide in the mixture,
the permeability of coal decreases till the point which the composition of
mixture was approximately 60% CO2 and 40% CH4 and then begun to increase.Finally
they explained the adsorption effect on the permeability of coal to methane
and the carbon dioxide mixture by defining the mutual and individual adsorption
effects, which are the effects of adsorption of a gas from a mixture and
from an individual gas on coal permeability, respectively.
|